Imaging Machine : automated microscopy
The Imaging Machine (IM) is a fully automated widefield microscope, for brightfield and fluorescence imaging of various samples.
It is an ideal platform for cell-based high-content screening assay or phenotypic screening with small-model organisms (see the Gallery for examples).
Its static sample holder, combined with a mobile optical unit, prevents any perturbation while imaging motion-sensitive samples, such as non-adherent cell-cultures. Moreover, the built-in temperature regulation ensures perfect imaging conditions for long time-lapse imaging.
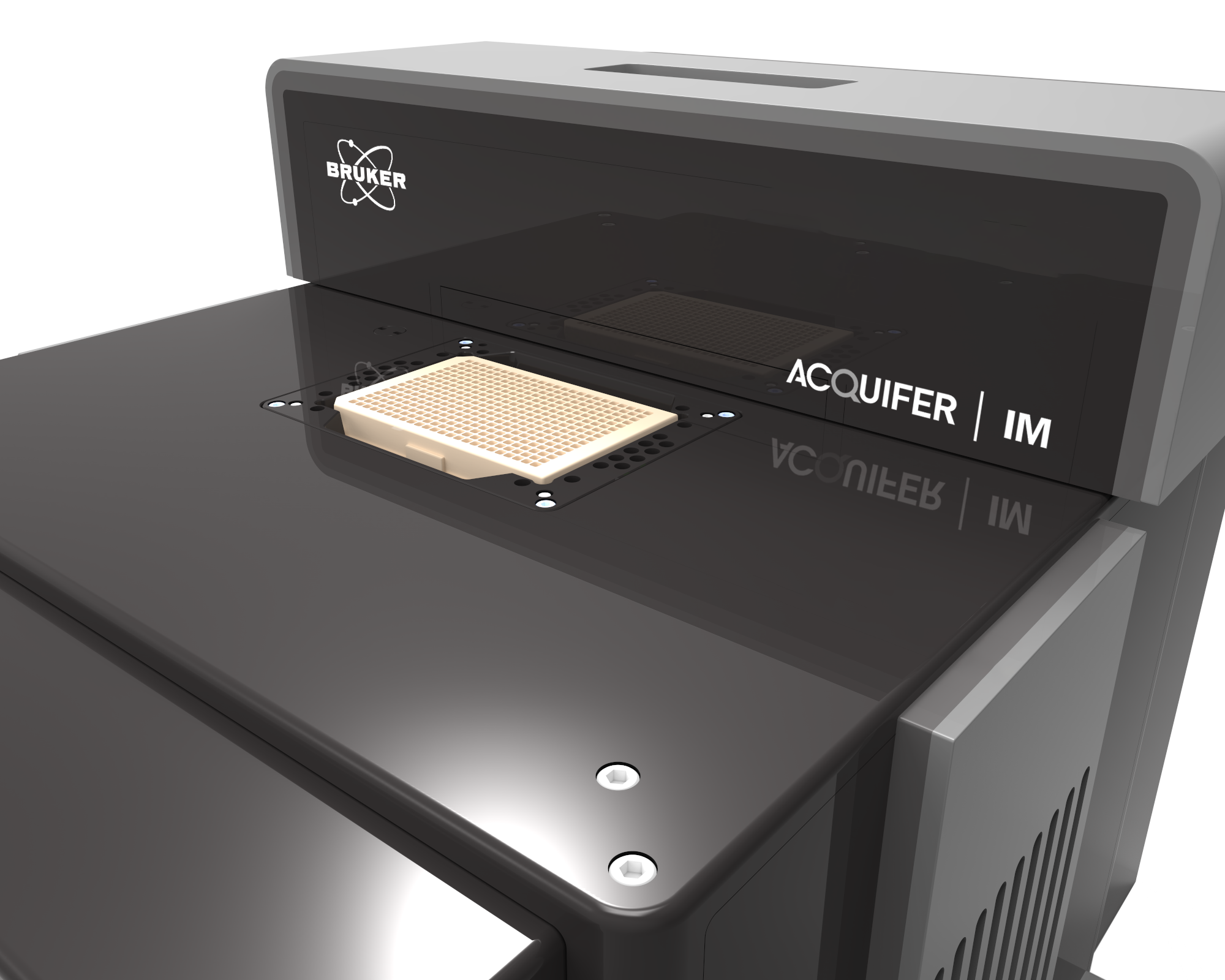
The Imaging Machine (IM)
Fully-automated widefield microscope (brightfield/fluorescence) for your screening/high-throughput imaging assay
- High positioning resolution and accuracy
- Steady sample position guaranteed by immobilized sample-holder and moving optics
- Built-in temperature regulation for time-lapse imaging
- Data storage & processing integrated
- Robotic lid and open-interface (TCP/IP) for integration in automated workflows
- Image-processing extensions for common open-source software (ImageJ/Fiji…)
- Optional module for photomanipulation
The IM software streamlines multi-dimensional imaging for multiple samples in well-plates or slides.
Define once your plate-layout, and image-dimensions (Channel, Z-slices, Time), optimize your acquisition parameters for one or a few positions, then start the full-plate imaging.
Save/load your settings as presets for routine execution.
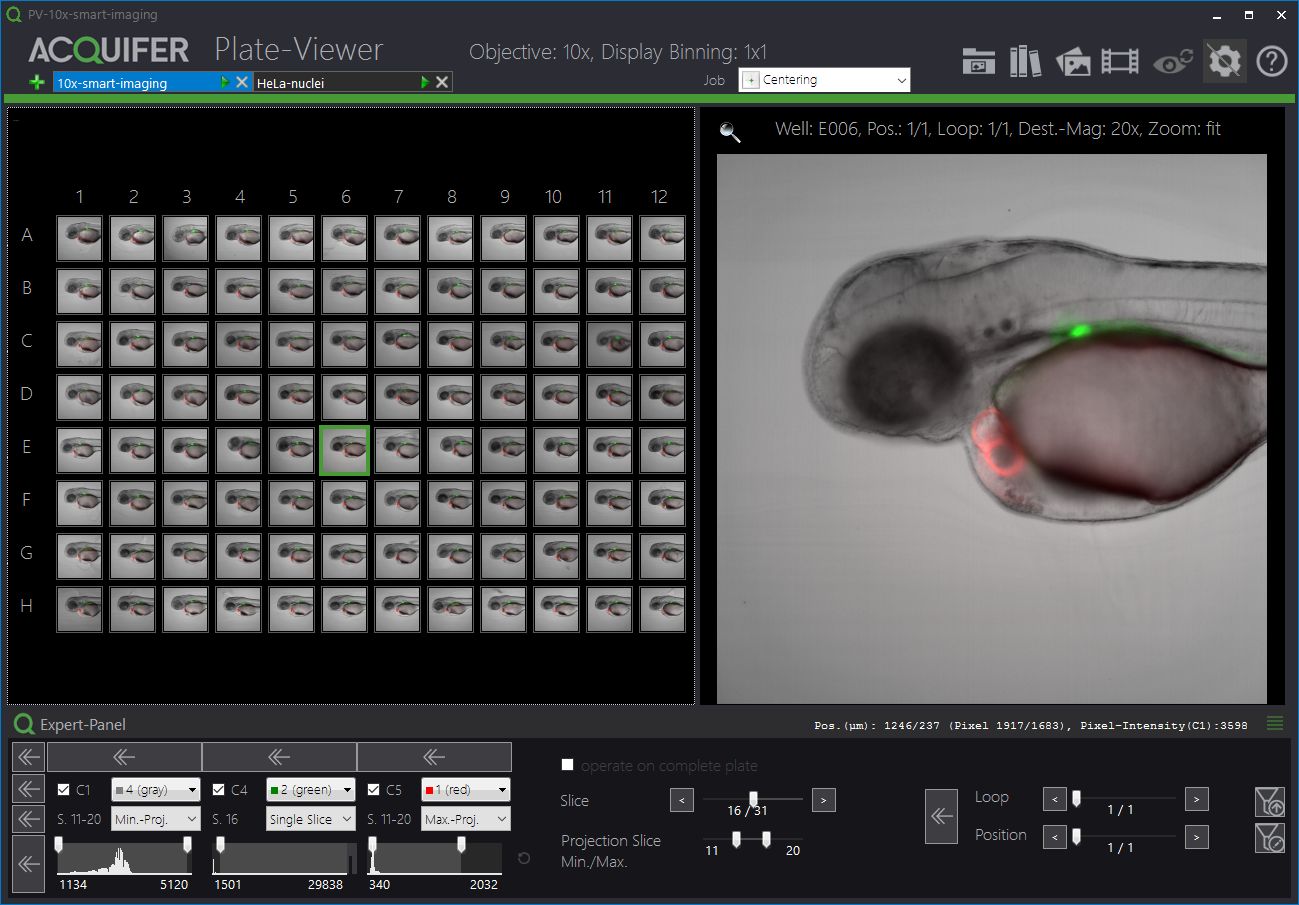
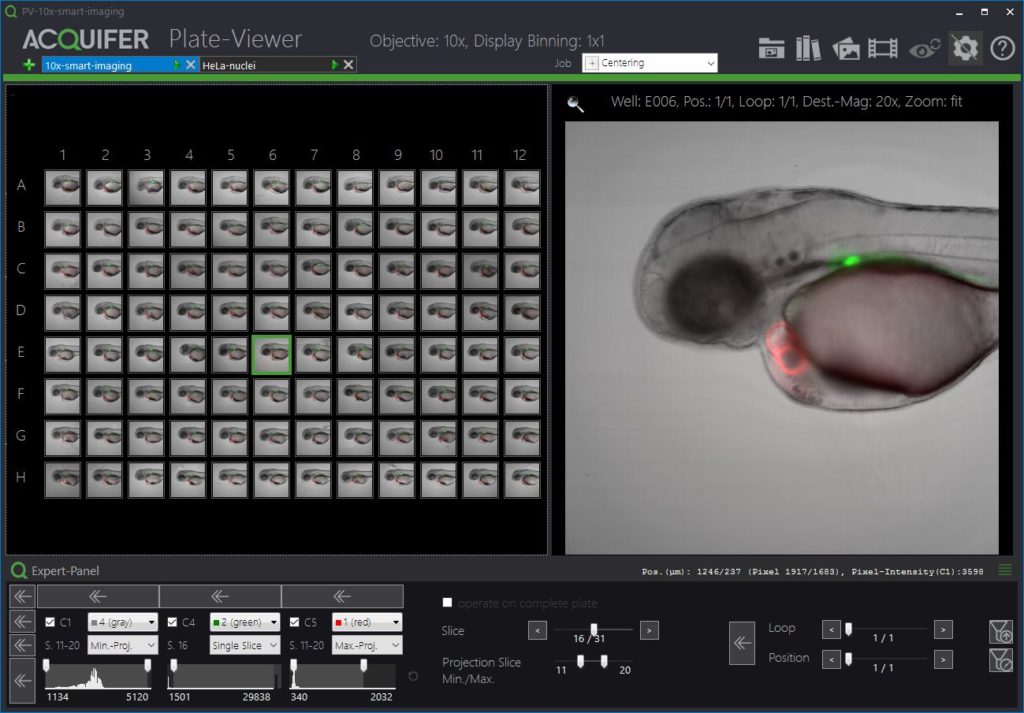
The Plate-Viewer
The Plate-Viewer is our visualization software for the IM, facilitating the parallel inspection of acquired images, and helping with the conduction of higher-resolution imaging-workflows.
Applications
ZEBRAFISH
...learn more about our zebrafish applications
- Dorsal orientation tool
- Lateral orientation tool
- Image optimization – Deconvolution
- Visualization tools
(TIME-LAPSE) CELL BASED
- Optimized Temperature control – unique laminar flow design for MT plates
- Light power sensor at sample level
- precise re-positioning (linear motor axis + 1nm resolution encoder)
- Laser Autofocus
YEAST
- Static MT plate holder (for very force sensitive yeast lines)
- Laser Autofocus
- Yeast optimized image based auto-focus
- Image processing workflows
LARGE PROJECTS
- Designed for single and multi Imaging Machine projects
- HIVE Data module integration allows seamless project scaling
- Light power, temperature, motor sensors designed for optimized data reproducibility
- Benchtop format for minimal lab space usage
An easy-to-use tool for visualizing high content screening data and supervised feedback microscopy
The ACQUIFER Plate-Viewer software is an easy-to-use gateway to large-scale image datasets acquired with the ACQUIFER Imaging Machine. It provides an intuitive overview of screening data in combination with functionalities to enhance image features, save data visualizations or time-lapse movies as well as supervised feedback microscopy functionalities, i.e. the selection of region of interest to be subsequently centred on the ACQUIFER Imaging Machine.
Key Features
- Intuitive visualization of multidimensional screening datasets.
- Easy browsing of well coordinates, z-slices, timepoints and subpositions.
- Display of single slices or z-projections (min and max projection).
- Look-Up tables and histogram adjustments.
- Data export of single images, z-projections, z-stacks or time-series in various standard file formats (tif, jpg, png, bmp, mp4).
- Select regions of interest for feedback microscopy using the built-in click-tool or automated object detection.
- Modify ACQUIFER Imaging Machine scripts to conduct pre-scan / re-scan experiments, e.g. zoom-in on regions of interest.
- For advanced users: generate plugins to process images with various software packages (e.g. Fiji/ImageJ…).
Technical Specifications IM
| Z axis range | 25 mm, 1nm resolution, linear motor , absolute encoded Z axis design optimized for speed and precision (for data deconvolution) |
| XY axis range | Linear Motors with absolute position encoder, 1nm resolution 120 mm x 80 mm, 1nm resolution, linear motor, absolute encoded |
| typical LED lifetime | > 15.000 hours on-time |
| Objectives | 2x, 4x, 10x, 20x, 40x (Nikon) |
| Mechanical design | Static sample holder, moving optics block Linear Motors with absolute position encoder, 1nm resolution |
| LED power sensor | Power Sensor at MT plate level |
| LED light wavelengths | 385, 405, 455, 470, 505, 528, 595, 625 nm |
| LED light source | Omicron LED-HUB , up to 6 modules parallel, temperature controlled |
| Fluo Filter sets mono | any 25mm “Semrock” combination – see Semrock-Fluo-table |
| Fluo Filter sets duo-quad | in combination with LED light source module for ultra fast switching |
| Fluo Filter blocks | custom type, up to 5 |
| Camera | Hamamatsu sCMOS 2k x 2k |
| Temperature + CO2 | |
| CO2 | via external controller |
| Incubation type | robotic lid + integrated laminar flow, pressure free |
| Incubation range | 20°C – 40°C * if T > ambient temp +3 °C |
| Remote control | optional via TCP based commands from any software |
| Focussing | Laser Autofocus @780nm and image based Autofocus (standard, yeast) |
| Power | 120V - 240V |
| Ambient conditions | 18°-25°C / 65° – 77 ° F (Indoor use), Relative humidity 30%-80% (without condensation) |
| Weight | 62 kg |
| H x W x D | 553mm x 528mm x 555mm |
Downloads
THE ACQUIFER IMAGING MACHINE
AN AUTOMATED MICROSCOPY PLATFORM FOR HIGH-THROUGHPUT SCREENING OF BUDDING YEAST
The budding yeast Saccharomyces cerevisiae, an eukaryotic microorganism, is a powerful model organism to address biomedical research questions on the genome-scale using growth or biochemical assays. This is complemented by large-scale imaging screens using automated high-throughput microscopy to visualize fluorescent reporter localizations. This allows monitoring the full yeast proteome via GFP fusion proteins or any phenotype that can be followed by a fluorescent marker. Due to the small cell sizes, photosensitivity and non- adherence of yeast cells, these high throughput screening assays demand advanced automated imaging platforms that are capable of robustly and reproducibly acquiring high-resolution datasets for visualization and scoring of cellular and sub-cellular phenotypes.
ACQUIFER SMART IMAGING
AN INTERFACE FOR REMOTE CONTROLLING AND UNSUPERVISED FEEDBACK MICROSCOPY ON THE IMAGING MACHINE
High content screening is routinely employed to automatically acquire multi-dimensional image datasets at fixed positions within wells of microtiter plates. While this is sufficient for many applications, it imposes rather inflexible screening workflows as imaging positions are pre-defined by users, often regardless of specimen location or sample characteristics. This can lead to acquisition of unnecessary datasets, omission of features of interest and could hinder more complex assays that would depend on real-time analysis of image data.
THE ACQUIFER PLATEVIEWER
A TOOL FOR VISUALIZING HIGH CONTENT SCREENING DATA AND SUPERVISED FEEDBACK MICROSCOPY
Modern High Content Screening microscopes allow rapid automated imaging of entire microtiter plates by imaging fixed positions within each well. This is ideal for in-vitro cell culture based readouts or other assays with evenly distributed phenotypes. However, it imposes limitations when large specimen or rare events are studied. The limited field of view of objectives often force users to utilize low magnification lenses leading to low resolution data or accept the omission of features of interest in many wells.
BROCHURES

Why the Imaging Machine ?
We developed our Imaging Machine for easy, precise, robust and smart high content screening applications without the need for an expert to manage the experiment. We focused on integrated data management, long term data transparency, sensitive samples and a robust machine technology. In other words: Imaging Machines are designed for everyone to use.