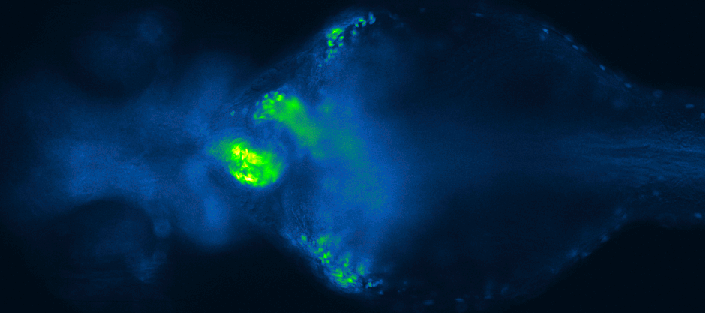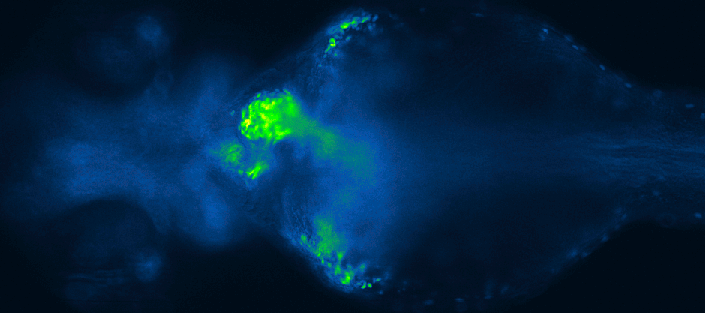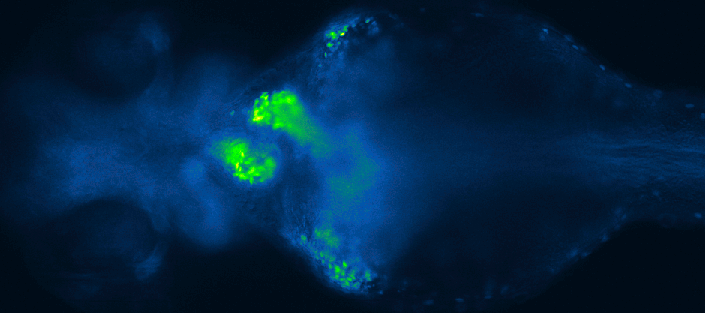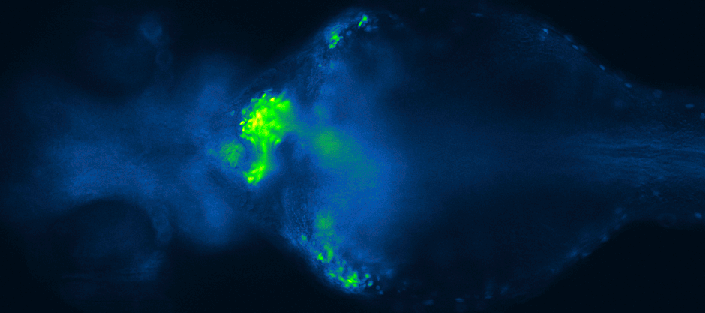
Zebrafish microscopy imaging
Automated microscopy for zebrafish and other whole-organisms screening assay
ACQUIFER is your partner for zebrafish research and imaging. We have a well-established experience in microscopy imaging of zebrafish larvae and other small model-organisms, covering the full experimental workflow from sample mounting, complex imaging to image-analysis.
The ACQUIFER Imaging Machine is the ideal platform for high content screening with such animal models.
It provides highly-stable imaging conditions for time-lapse imaging of non-adherent and motion-sensitive specimen, thanks to its unique opto-mechanical design featuring moving-optics and a static stage.
Besides, the integrated environment control assures optimal in-vivo conditions for image-based screening or long-term observation of multiple developing specimen.
Our latest innovation, the photomanipulation-module streamlines the parallel conduction of laser-ablation, photo-activation or fluorescence lifetime imaging experiments.
Finally, in combination with the ACQUIFER PlateViewer and the script-based Smart Imaging interface, the Imaging Machine enables challenging whole-organism screening workflows.
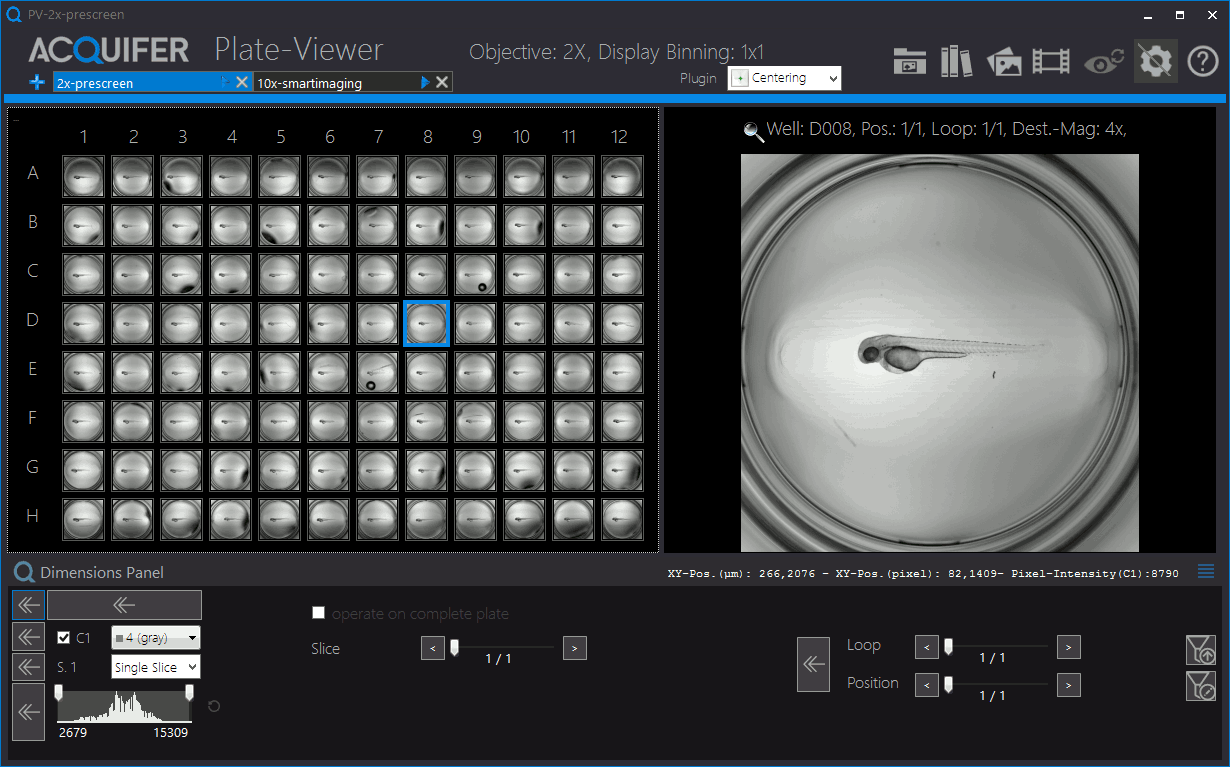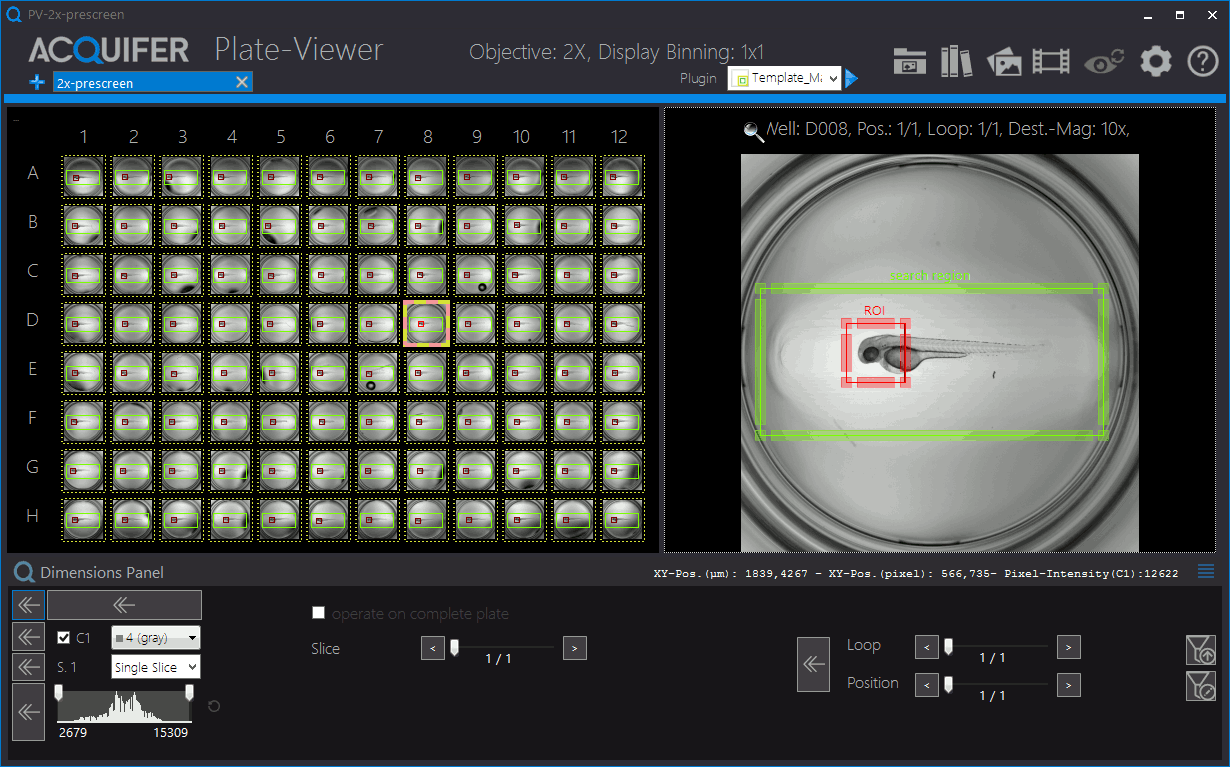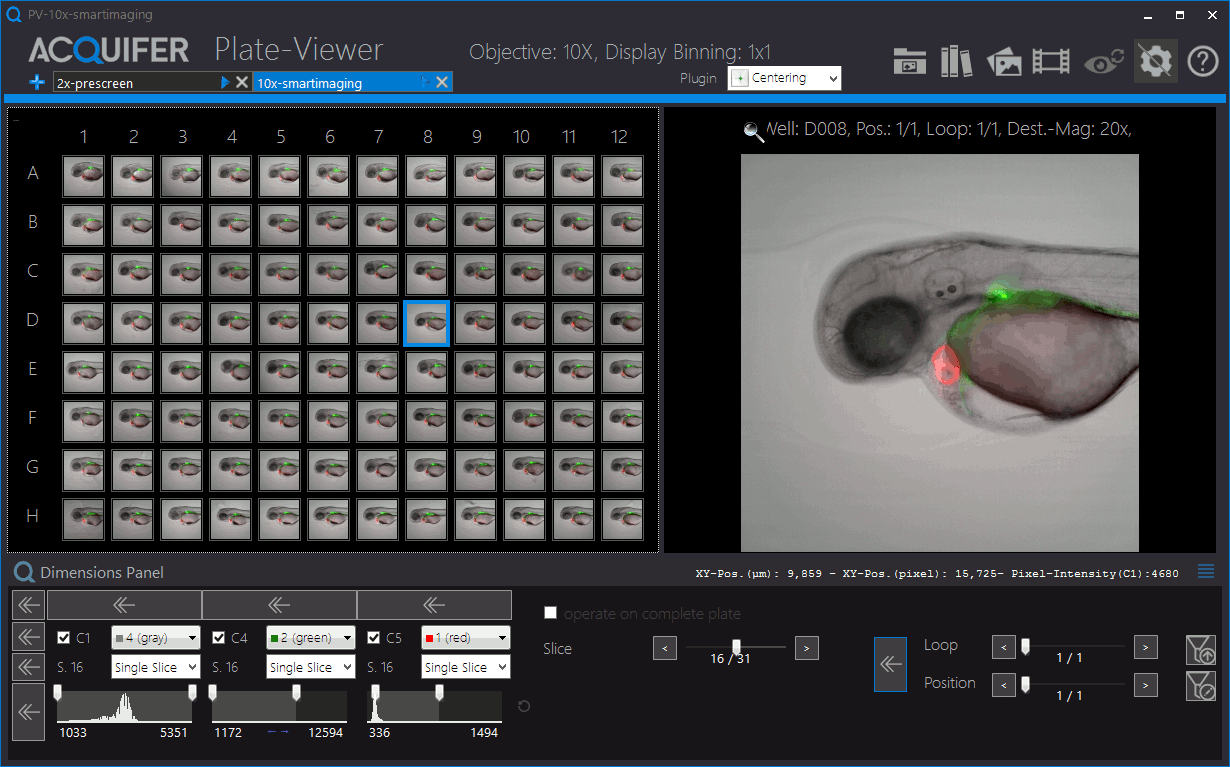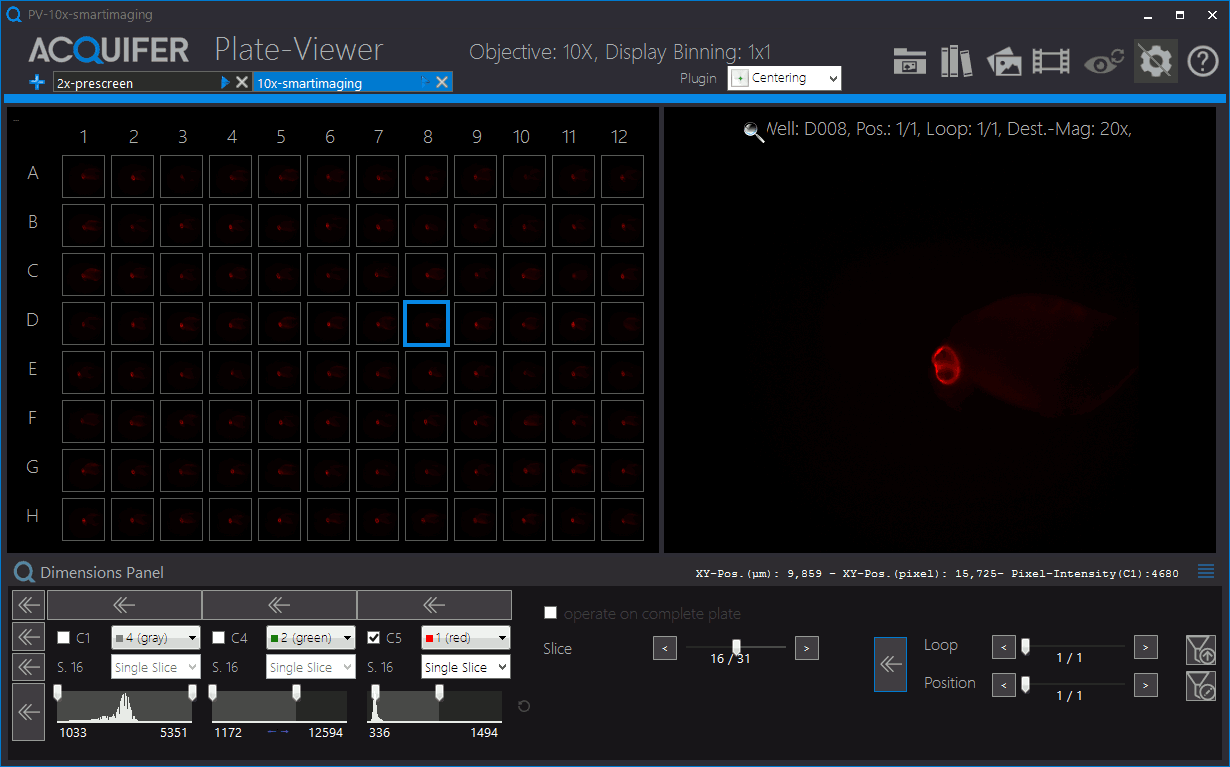
Shown is an animation of a typical zebrafish workflow for organ-specific screening, and workflow control and visualization in the PlateViewer. A pre-screen at low resolution (2x objective) is followed by automated region of interested detection, re-centering of target regions and multi-dimensional image acquisition at higher magnification (10x). Images by Eleonora Lupi.
Example Applications and Assays
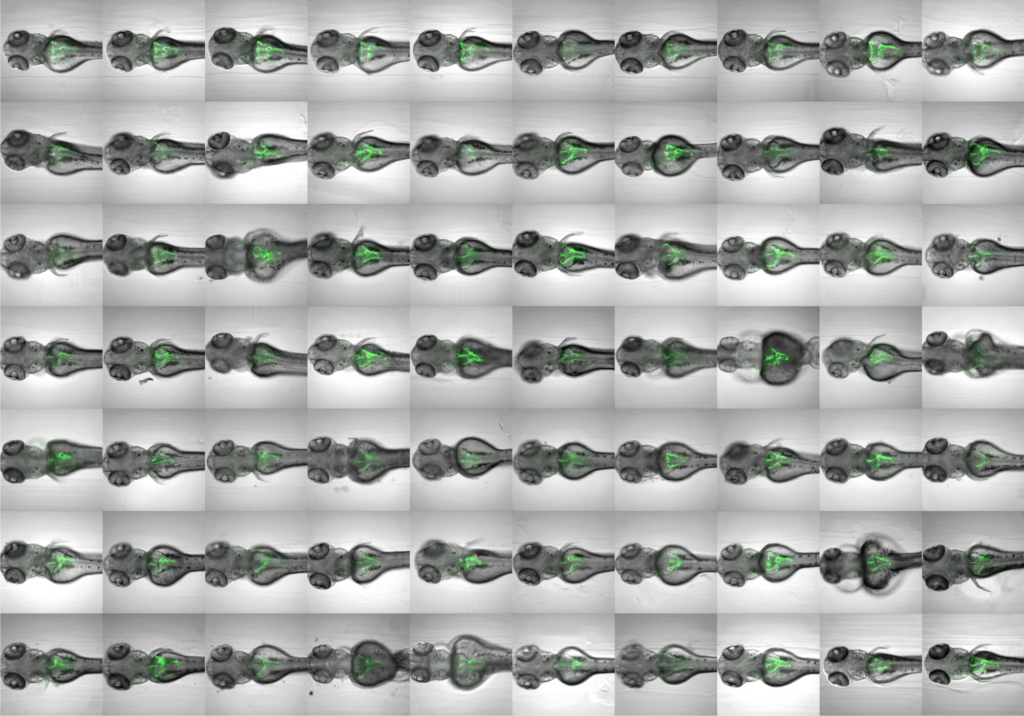
Organ-specific screening in cystic kidney zebrafish disease models. See also: Pandey et al. (2019).

High content screening in zebrafish for developmental nephrotoxicity of approved drugs. Click image to watch animation. See also: Westhoff et al (2020).

Screening for chemical modulators of heart development in zebrafish. Images by 4DHeart ESR Eleonora Lupi (Nadia Mercader lab, University of Bern & Acquifer).
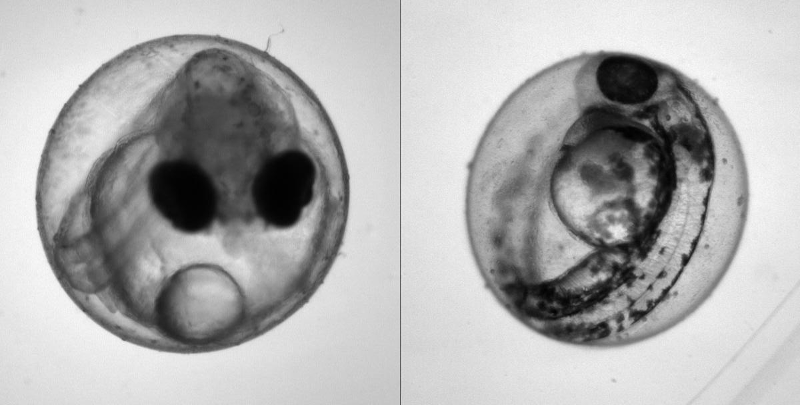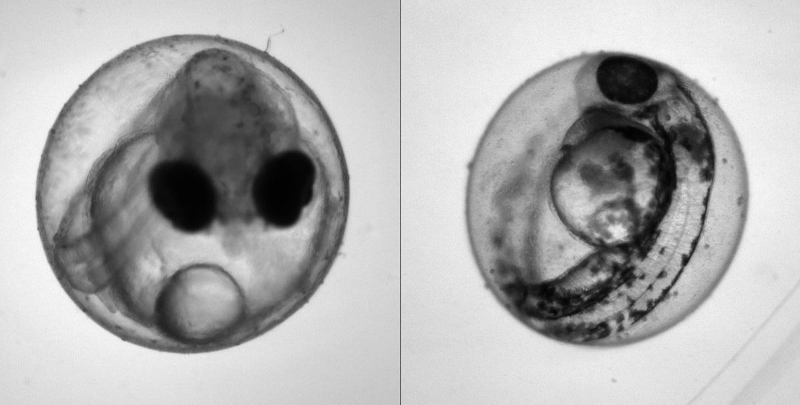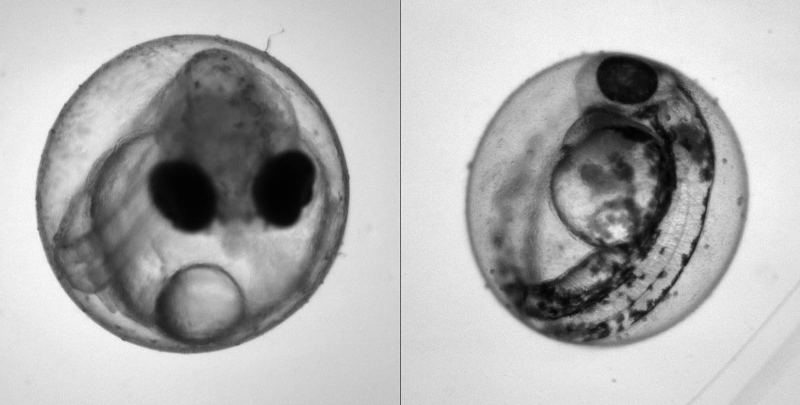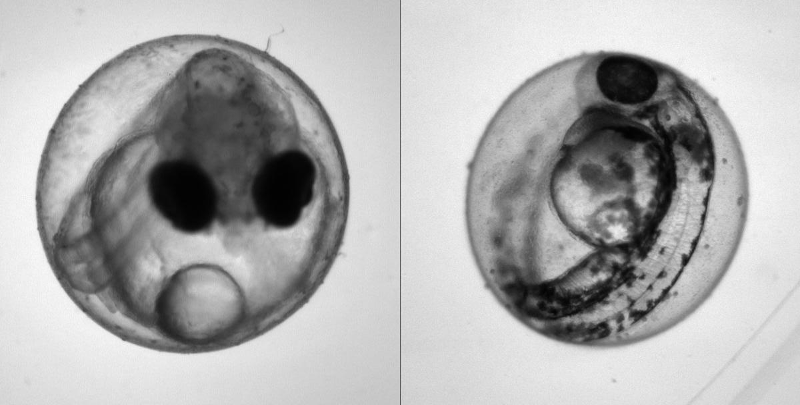
Heartbeat quantification in medaka and zebrafish embryos under physiological conditions. See also Gierten et al. (2020).
Assessment of renal function in larval zebrafish. See also Steenbergen et al. (2020).
Embryonic zebrafish xenograft assay of cancer metastasis – Image courtesy of Arwin Groenewoud (Ewa Snaar-Jagalska lab, Leiden University).
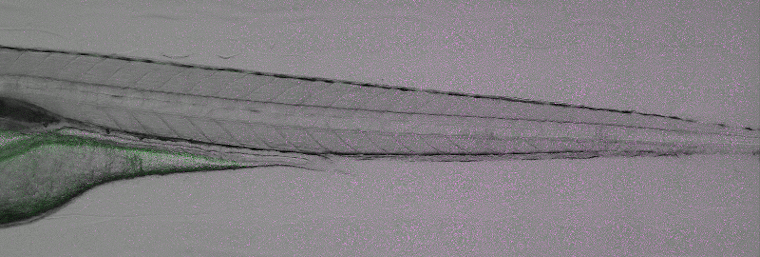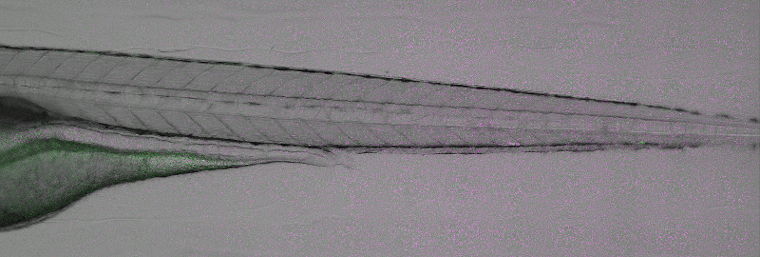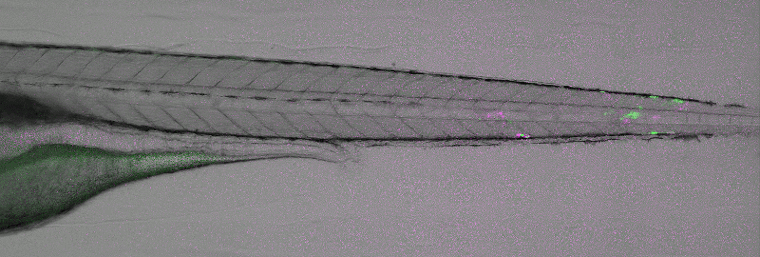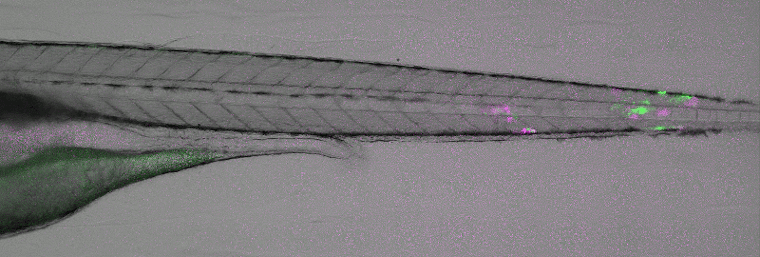
Disease modelling of viral infections. Image courtesy of ImageInLife ESR Valerio Laghi (Jean-Pierre Levraud lab, Institut Pasteur, Paris).
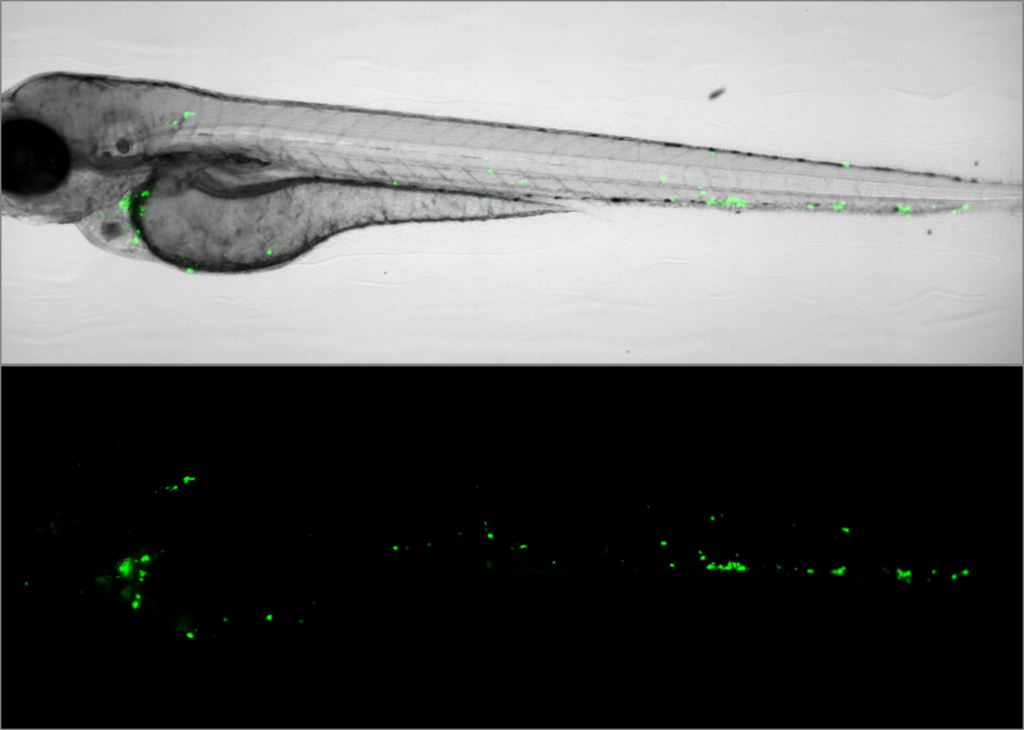
Bacterial burden assay in zebrafish. Image courtesy of ImageInLife ESR Salomé Muñoz Sánchez (Annemarie Meijer Lab, Leiden University).
Time-lapse imaging to characterize different anesthesia conditions in medaka. Click image to watch movie. See also Lischik et al. (2019).

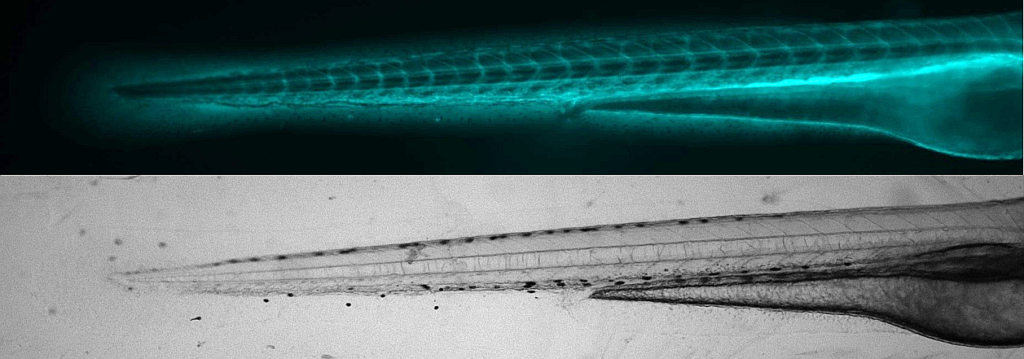
Tail cut assays to study inflammation in zebrafish. Image courtesy of Yufei Xie, Mahmoud Halima (Annemarie Meijer and Marcel Schaaf labs, Leiden University).
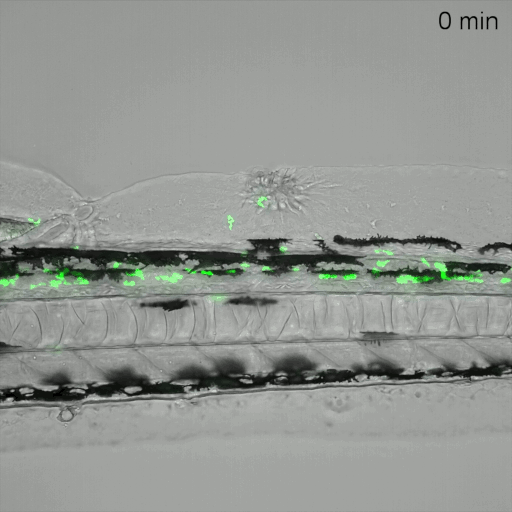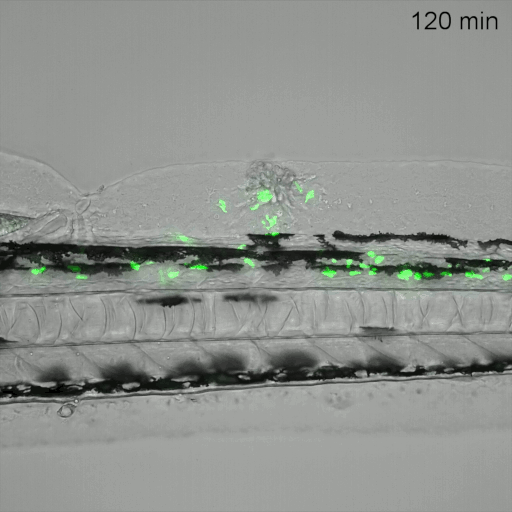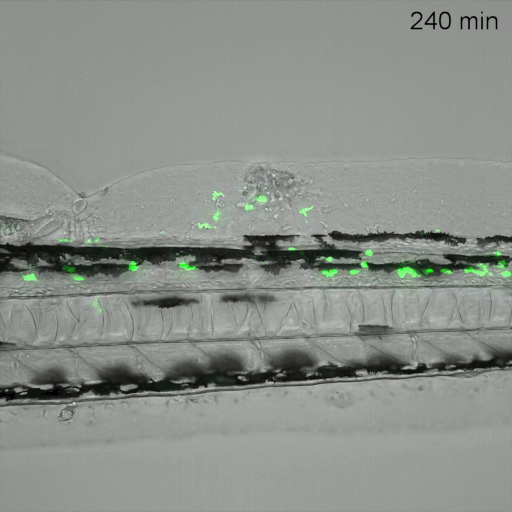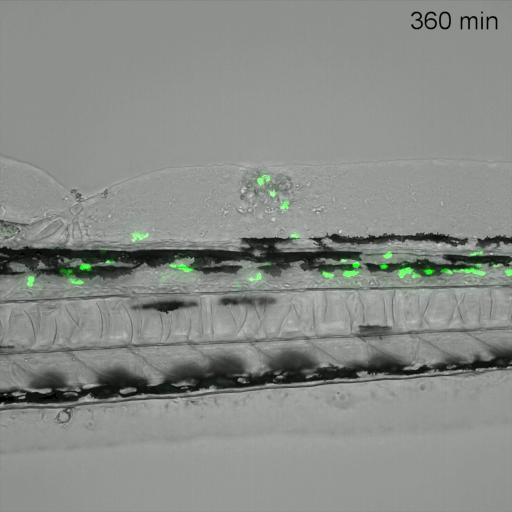
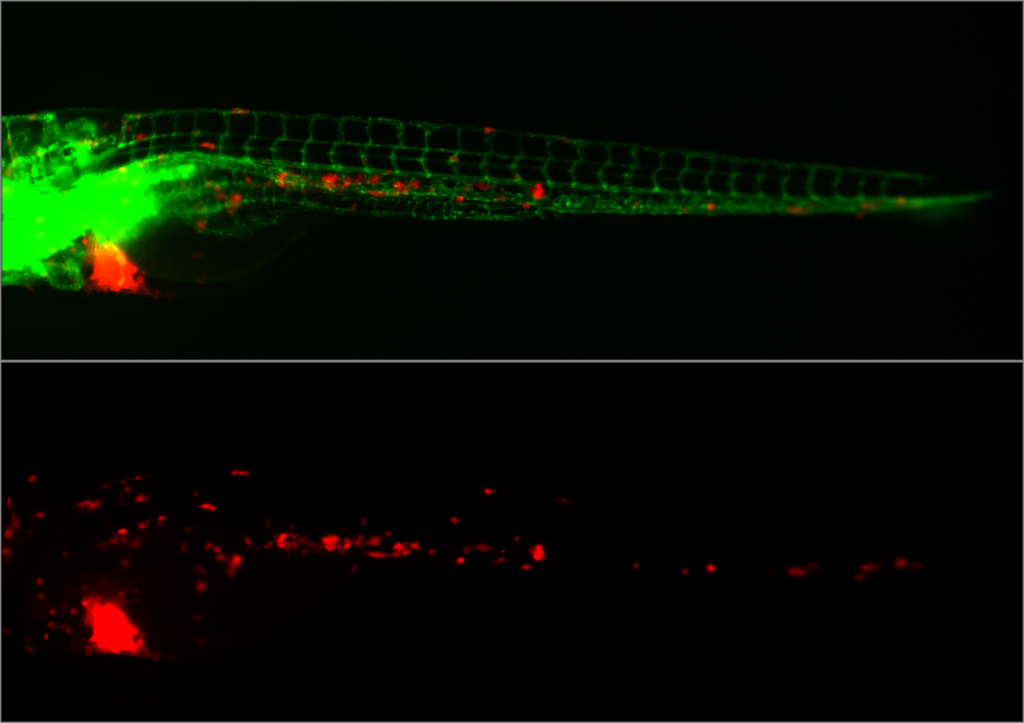
Monitoring of neutrophil migration after automated laser injury. Image data generated by INFLANET ESRs Nils Olijhoek (Stephen Renshaw and Phil Elks labs, University of Sheffield) and Sankeert Satheesan (ACQUIFER)
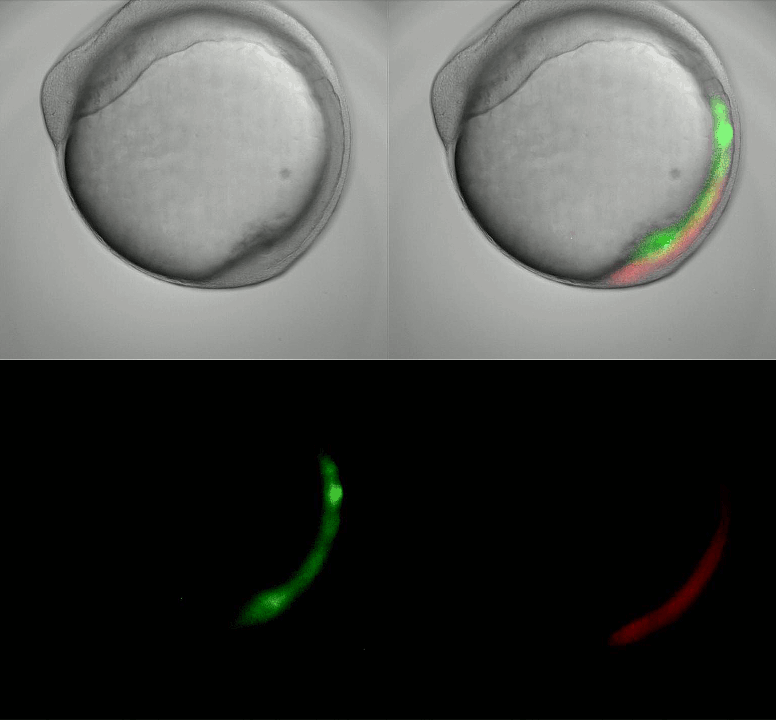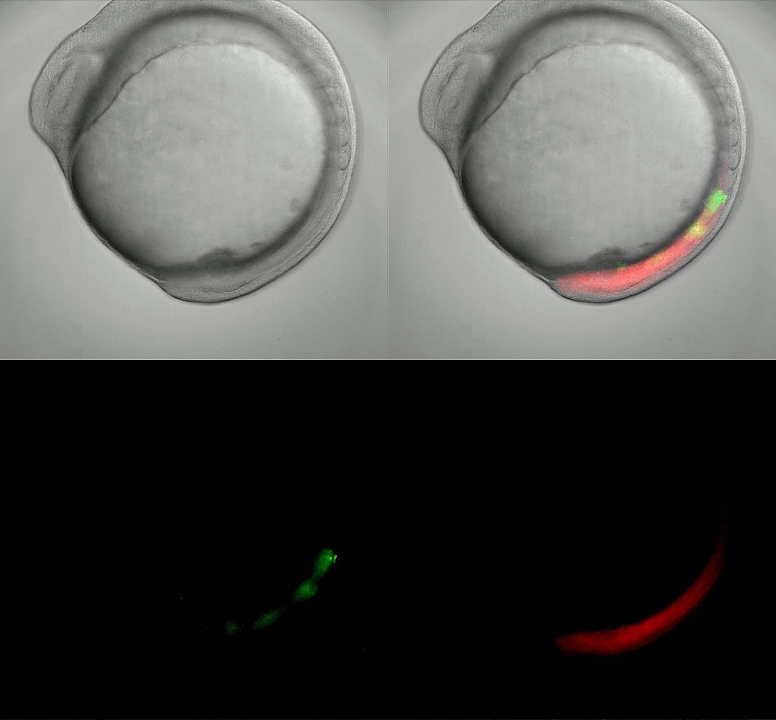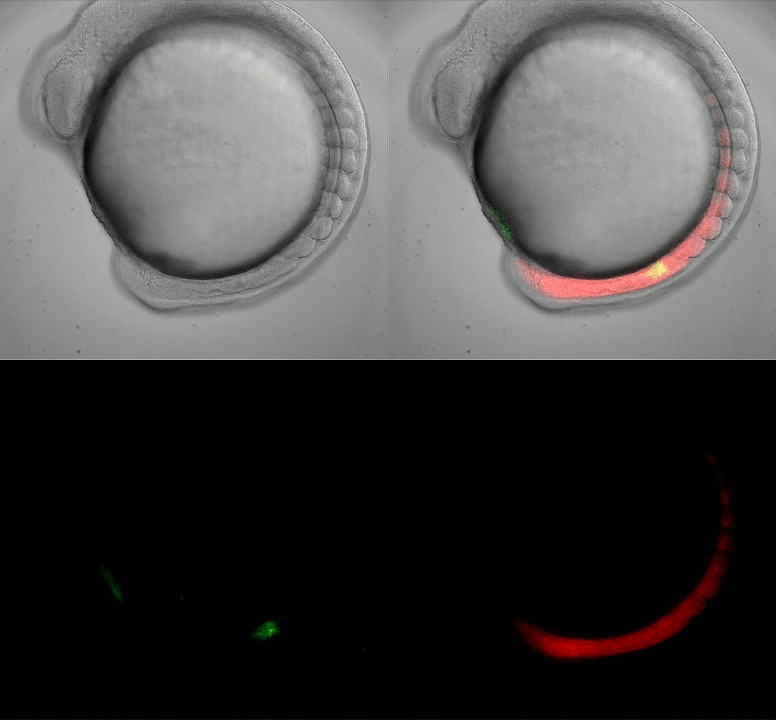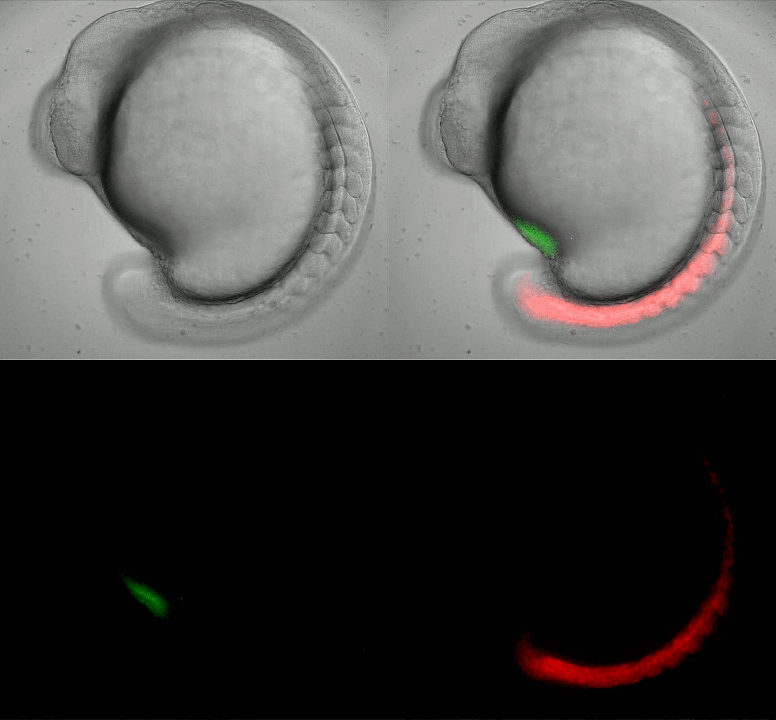
Imaging oscillating reporter gene expression to study pattern formation in zebrafish segmentation. Image courtesy of Daniele Soroldoni and Andy Oates lab, EPFL.